Phosphorus
Phosphorus provides a nutrient or food source for algae. Phosphorus combined with inorganic nitrogen poses serious pollution threats to receiving waters because of high algae growths which result from the presence of the two nutrients in water. Algae in water are considered unsightly and can cause tastes and odors in drinking water supplies. Dead and decaying algae can cause serious oxygen depletion problems in receiving streams which in turn can kill fish and other aquatic wildlife.
By removing phosphorus in the effluent of a wastewater treatment plant, the lake or river that the treatment plant discharges into will have one less nutrient that is essential for algae growth. This reduction in an essential nutrient reduces the growth of the algae.
The U.S. Environmental Protection agency and other water quality regulating agencies recognize the need to protect rivers and lakes from excessive growths of algae. Because of this, the agencies are requiring that wastewater treatment plants remove phosphorus in the effluent in order to protect the river or stream by eliminating a nutrient that can cause algae growth.
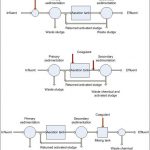
Lime precipitation, luxury uptake, and filtration following aluminum sulfate flocculation are the most common types of phosphorus removal systems.
When lime (calcium hydroxide (Ca(OH)2) is mixed with effluent from a wastewater treatment plant in sufficient concentration to bring about high pH in the water, a chemical compound is formed which consists of phosphorus, calcium and the hydroxyl (OH-) ion. This compound can be flocculated or combined in such a way as to form heavier solids which can settle in a clarifier for phosphorus removal. A substantial amount of the lime reacts with the alkalinity of the wastewater to form a calcium carbonate precipitate which also settles out with the phosphorus sludge.
There are three general physical or chemical reactions which take place during lime precipitation for phosphorus removal.
1. Coagulation. When chemicals are added to wastewater, the result may be a reduction in the electromagnetic forces which tend to keep suspended particles apart. After chemical addition, the electrical charge on the particles is altered so that the suspended particles containing phosphorous, tend to come together rather than remain apart.
2. Flocculation. Flocculation occurs after coagulation and consists of the collection or agglomeration of the suspended material into larger particles. Gravity causes these larger particles to settle.
3. Sedimentation. As discussed in previous chapters on primary and secondary clarification methods, sedimentation is simply the settling of heavy suspended solid material in the wastewater due to gravity. The suspended solids which settle to the bottom of clarifiers can then be removed by pumping and other collection mechanisms.
Lime precipitation for phosphorus removal requires lime feeding systems, mixing and flocculation areas, chemical clarifiers for sedimentation and the proper pumps and piping for removal of lime phosphorus sludge. Other equipment includes facilities for pH adjustment of the effluent, recovery of the lime, and disposal of the phosphorus sludge.