Breakpoint Chlorination
Since ammonia is present in all domestic wastewaters, the reaction of ammonia with chlorine is a great significance. When chlorine is added to waters containing ammonia, the ammonia reacts with hypochlorous acid (HOCl) to form monochloramine, dichloramine and trichloramine. The formation of these chloramines depends on the pH of the solution and the initial chlorine-ammonia ratio.
Ammonia + Hypochlorous -> Acid Chloramine + Water
NH3 + HOCl ‡ NH2Cl + H2O monochloramine
NH2Cl + HOCl ‡ NHCl2 + H2O dichloramine
NHCl2 + HOCl ‡ NCl3 + H2O trichloramine
In general at the pH levels that are usually found in wastewater (pH 6.5 to 7.5), monochloramine and dichloramine exist together. At pH levels below 5.5, dichloramine exists by itself. Below pH 4.0, trichloramine is the only compound found. The mono-and dichloramine forms have definite disinfection powers and are of interest in the measurement of chlorine residuals. Dichloramine has a more effective disinfecting power than monochloramine.
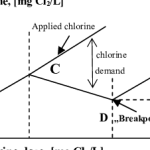
If enough chlorine is added to react with the inorganic compounds and nitrogenous compounds, then this chlorine will react with organic matter to produce chloro-organic compounds or other combined forms of chlorine, which have a slight disinfecting action. Then if enough chlorine is added to react with all the above compounds, any additional chlorine will exist as Free Available Chlorine, which has the highest disinfecting action. The term ‘Breakpoint Chlorination” refers to the breakpoint shown below.
The Breakpoint Curve shown above illustrates the formation and destruction of chloramines before free residuals are achieved. Every system’s breakpoint will vary depending on the chemical makeup and chlorine demand of the raw wastewater.
As chlorine is added to the water, it reacts with the ammonia that is present and a combined residual reading is obtained (A). In this case, as the dosage increases to about 2 ppm (mg/l) the combined residual drops because the chloramines are being destroyed (C). When the dosage reaches 3 ppm (mg/l), the breakpoint occurs and first free chlorine residual is obtained. Once the breakpoint has been reached, the free residual will increase at the same rate as the dosage (D). There may still be some combined residual in the water even though the breakpoint has been reached, but it will remain at this minimum level as long as the dosage is greater than 3 ppm (mg/l).
There are three methods that are used to test water for chlorine residual. Amperometric titration provides for the most convenient and the most repeatable results; however, the equipment costs more than equipment for the other methods. The Amperometric titration is most often used in the laboratory. Two other methods are field tests – the Ortho-Tolidine-Arsenite (OTA) test, which was the industry standard until the mid -1970’s, and the DPD method. Problems were found with the OTA test in that iron and nitrites in the water would interfere with the test. In addition, OTA was found to be a carcinogen. It is no longer used for chlorine residual testing today. Instead, the Diethyl-p-Phenylene-Diemine (DPD) test is used for fieldwork.
The DPD test is a colormetric analysis. The reagent is added to a vile (cuvette) of sample water. Another vile of sample water serves as a “blank.” If chlorine is present the sample will turn pink or red. The blank is placed in front of the “color wheel” and the sample is compared to the color wheel and blank. There are two chemical packets for the DPD test. One is used for free chlorine and the other is used for total chlorine residual. Subtracting the free residual from the total residual will give you the combined residual.